
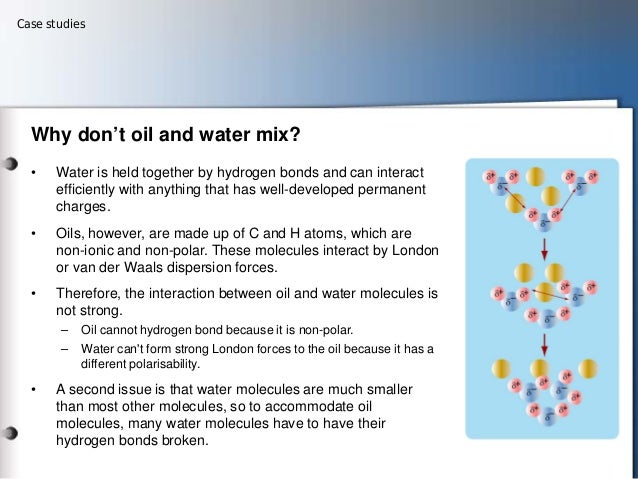

Oil and water do not mix – the mantra is familiar to every college. You have to shake them to overcome the forces that hold the oil together. Now teachers may want to rewrite their lessons
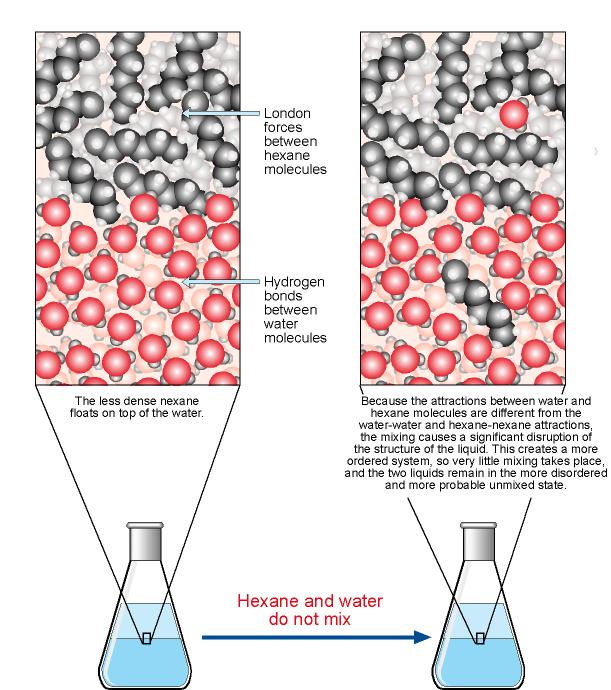
/123535151-56a12f683df78cf772683b2a.jpg)
What happens when Oil and Water mix? Have you tried to wash oily hands in just water and discovered that your hands stayed oily? Or think of crude oil that spills from a tanker in the ocean and floats on top of the water.
Why Oil and Water Don’t Mix. September 30, 2015 Melissa Leave a comment. Alex S. asks: Why doesn’t oil and water mix? they knit tightly together with hydrogen bonds. In fact, oil floats on water because it is less dense, with these super-tight hydrogen bonds between water molecules holding them closer together than the bonds between the


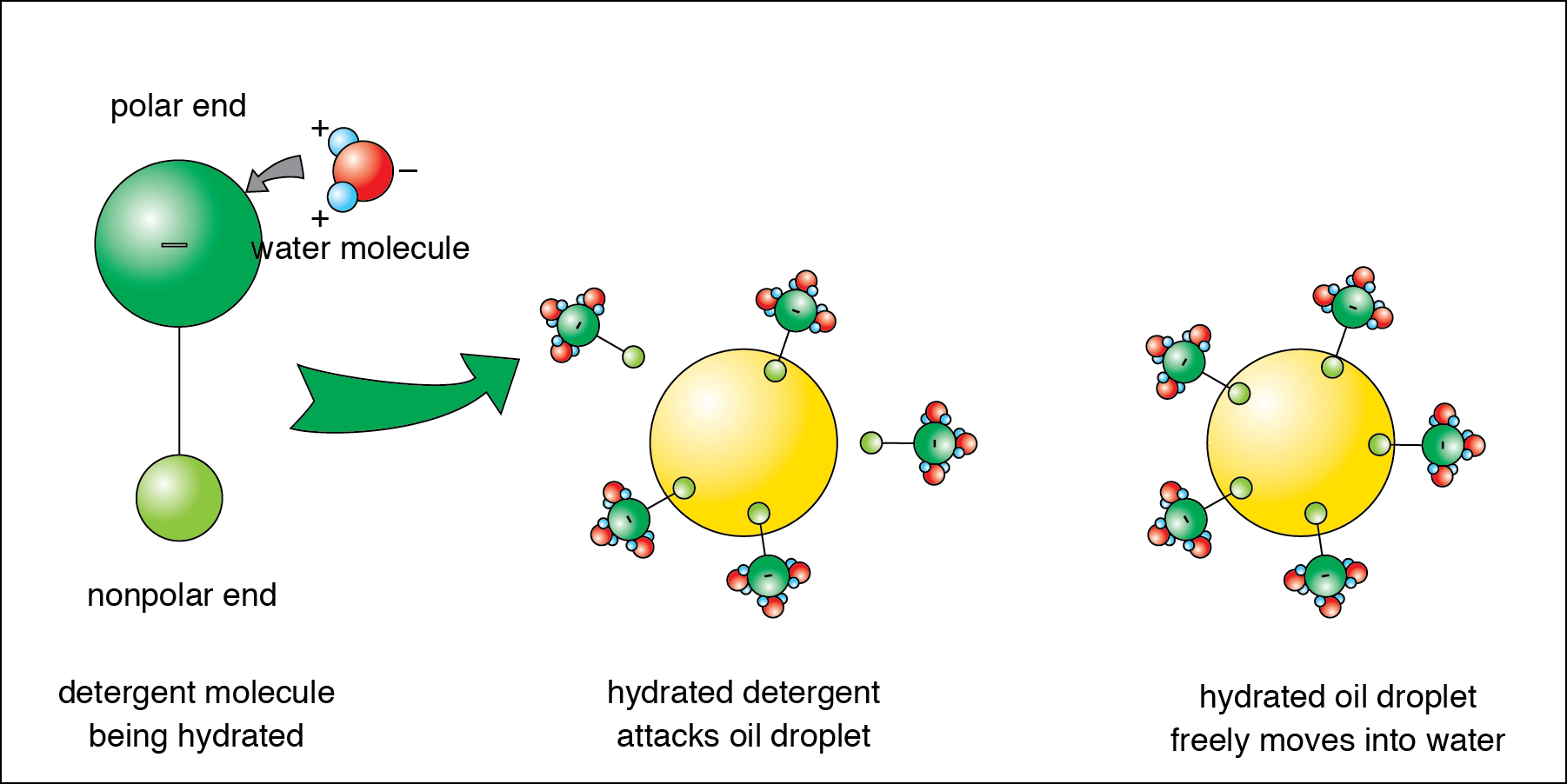
This explains why oil and water don’t mix. If you were to use emulsifier, you could get oil and water to mix together. An emulsifier is a substance that has one end that is …
Jun 15, 2006 · When a detergent is added and mixed up between the oil and the water, it holds hands with oil and water molecules and helps in getting the oil rinsed off with excess water. Such a mixture where oil and water can finally be together with the help of another substance is called emulsion.
Density- Oil and water don’t mix well together because oil has a density of 0.91 to 0.93g/cm^3 while water has a density of 1.00g/cm^3 therefore as water is denser than oil,water sinks to the bottom while oil …

You can get oil and water to mix together if you add an emulsifier, which is a molecule with hydrophobic (repels water) and hydrophilic (attracts water) ends. Soap is an example of an emulsifier that can attract oil particles and suspend them in a way that allows them to mix with water particles.
Even when you mix oil and water together they still separate. The oil always floats to the top because it is less dense than water. Oil and water don’t mix because water molecules are more attracted to each other than to oil molecules.
The surfactants improve how well water can interact with a surface, while the emulsifiers help oil and water droplets mix together. Density and Immiscibility Oil floats on water because it is less dense or has a lower specific gravity, however, the immiscibility of oil and water …